Kineret make these agents more cost-effective alternatives to Ilaris for the treatment of sJIA and AOSD. Behandel een acute jichtaanval kortdurend met een hoge dosering orale NSAIDs orale glucocorticoïden of colchicine afhankelijk van de comorbiditeit en comedicatie van de patiënt.
 Preclinical Characterization And Clinical Development Of Ilaris Canakinumab For The Treatment Of Autoinflammatory Diseases Sciencedirect
Preclinical Characterization And Clinical Development Of Ilaris Canakinumab For The Treatment Of Autoinflammatory Diseases Sciencedirect
Placebo-treated patients 0 these infections were mainly related to the respiratory tract.

Ilaris vs kineret. For a small number of patients with asthma the incidence of serious infection was higher in Kineret-treated patients 45 vs. Indications for Prior Authorization. Treatment of latent tuberculosis infection should be initiated prior to therapy with Kineret.
Placebo-treated patients 0 these infections were mainly related to the respiratory tract. Kineret has been associated with an increased incidence of serious infections 18 vs. Where Ilaris canakinumab failed can Kineret anakinra succeed.
Swedish Orphan Biovitrum AB Sobi is reporting that an investigator-initiated phase III study of its interleukin-1 IL-1 blocker Kineret in 594 hospitalized COVID-19 patients with moderate or severe pneumonia who had a poor prognosis uncovered what appear to be dramatic benefits. Learn about side effects interac. Biosimilars or Ilaris canakinumab subcutaneous injection also counts towards a trial of one other systemic agent for SJIA.
Kineret is used to treat the symptoms of moderate to severe rheumatoid arthritis Cryopyrin-Associated Periodic Syndromes Neonatal-Onset Multisystem Inflammatory Disease and Interleukin-1 Receptor Antagonist Deficiency. Pas medicatie aan op basis van intensieve monitoring van de ziekteactiviteit met als doel het bereiken van remissie of. Medicines called IL-1 blocking agents such as Kineret.
What is the most important information I should know about. ADMINISTRATION OF KINERETÒ SHOULD BE DISCONTINUED IF A PATIENT DEVELOPS A SERIOUS INFECTION. It is marketed by Swedish Orphan Biovitrum.
No significant change was found in Bath Ankylosing. For patients with body weight less than or equal to 40 kg the recommended dose is 2 mgkg administered every 4 weeks. The safety and effectiveness of Ilaris in treating these conditions were established in multiple clinical trials.
Patients in SJIA Study 1 received a single dose of ILARIS 4 mgkg n43 or placebo n41 via subcutaneous injection and were assessed at Day 15 for the efficacy endpoints and had a safety analysis up to Day 29. Gouty arthritis Two studies involving 454 patients with gouty arthritis showed that Ilaris was more effective than another anti-inflammatory medicine triamcinolone acetonide at reducing pain. Anakinra vergelijken met een ander geneesmiddel.
It is a recombinant and slightly modified version of the human interleukin 1 receptor antagonist protein. Compare Ilaris vs Kineret head-to-head with other drugs for uses ratings cost side effects and interactions. Placebo 07 in RA patients.
The study is planned to consist of three groups each comprising 18 patients. Anakinra brand name Kineret is a biopharmaceutical drug used to treat rheumatoid arthritis. Anakinra is administered at home by.
Placebo 07 in RA patients. Because of the similarities between childhood Stills disease and the adult form adult-onset Stills disease AOSD Ilaris is expected to have similar benefits in adults. Kineret has been associated with an increased incidence of serious infections 18 vs.
Start bij een vermoeden van reumatoïde artritis RA in de eerstelijnszorg met een NSAID en verwijs zo snel mogelijk naar de reumatoloog. The dose can be increased to 4 mgkg every 4 weeks if the clinical response is not adequate. The recommended dose of ILARIS for TRAPS HIDSMKD and FMF patients is based on body weight.
Canakinumab vergelijken met een ander geneesmiddel. Drugs that affect the immune system by blocking TNF have been associated with an increased risk of new tuberculosis and reactivation of latent tuberculosis TB. Stimuleer ter preventie van verergering van met jicht geassocieerde cardiovasculaire en metabole comorbiditeit een gezonde leefstijl.
For patients with body weight greater. 50 at Week 12 and 48 at Week 24. Inflammatory Conditions Kineret.
ILARIS with TNF inhibitors is not recommended because this may increase the risk of serious infections see Drug Interactions 71. The use of Ilaris in combination with other biologic. This is an open label controlled parallel group 3-arm multicenter study to assess the efficacy and safety of Emapalumab or Anakinra versus standard of care SoC.
Patients between 30 and 80 years will be eligible to participate in the study. Physician Package Insert Kineret anakinra Page 5 of 11 Amgen Thousand Oaks WARNINGS KINERETÒ HAS BEEN ASSOCIATED WITH AN INCREASED INCIDENCE OF SERIOUS INFECTIONS 2 VS. ILARIS i-LAHR-us canakinumab injection for subcutaneous use.
The safety of ILARIS compared to placebo in SJIA patients was investigated in two phase 3 studies see Clinical Studies 142. Patients should be evaluated for latent tuberculosis infection with a TB skin test. For a small number of patients with asthma the incidence of serious infection was higher in Kineret-treated patients 45 vs.
Anakinra Kineret SELF ADMINISTRATION. Behandel in de tweedelijnszorg volgens de treat-to-target-strategie.
 Current Mechanisms Of Il 1 Targeted Therapy Anakinra A Recombinant Download Scientific Diagram
Current Mechanisms Of Il 1 Targeted Therapy Anakinra A Recombinant Download Scientific Diagram
 Mechanism Of Action Of Anakinra Both Il 1a And Il 1b Act Through Il 1 Download Scientific Diagram
Mechanism Of Action Of Anakinra Both Il 1a And Il 1b Act Through Il 1 Download Scientific Diagram
Https Www Ema Europa Eu En Documents Variation Report Kineret H C 363 Ii 0056 Epar Assessment Report Variation En Pdf
 Figure 3 From Therapy Of Autoinflammatory Syndromes Semantic Scholar
Figure 3 From Therapy Of Autoinflammatory Syndromes Semantic Scholar
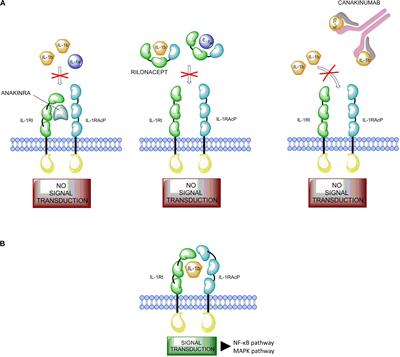 Frontiers Development And Role In Therapy Of Canakinumab In Adult Onset Still S Disease Pharmacology
Frontiers Development And Role In Therapy Of Canakinumab In Adult Onset Still S Disease Pharmacology
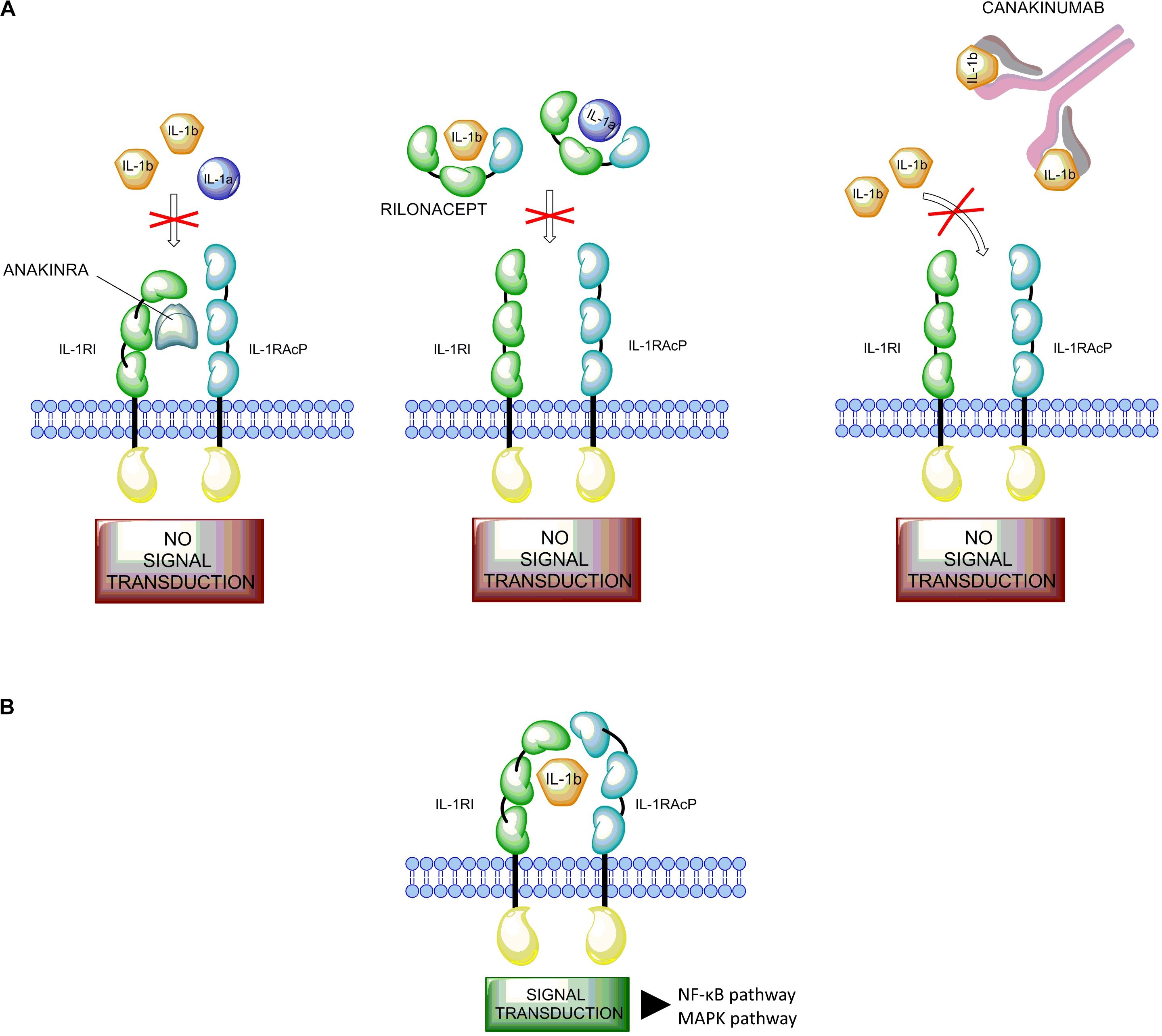 Frontiers Development And Role In Therapy Of Canakinumab In Adult Onset Still S Disease Pharmacology
Frontiers Development And Role In Therapy Of Canakinumab In Adult Onset Still S Disease Pharmacology
 Interleukin 1 Inhibitors That Are In Current Clinical Use Anakinra Is Download Scientific Diagram
Interleukin 1 Inhibitors That Are In Current Clinical Use Anakinra Is Download Scientific Diagram
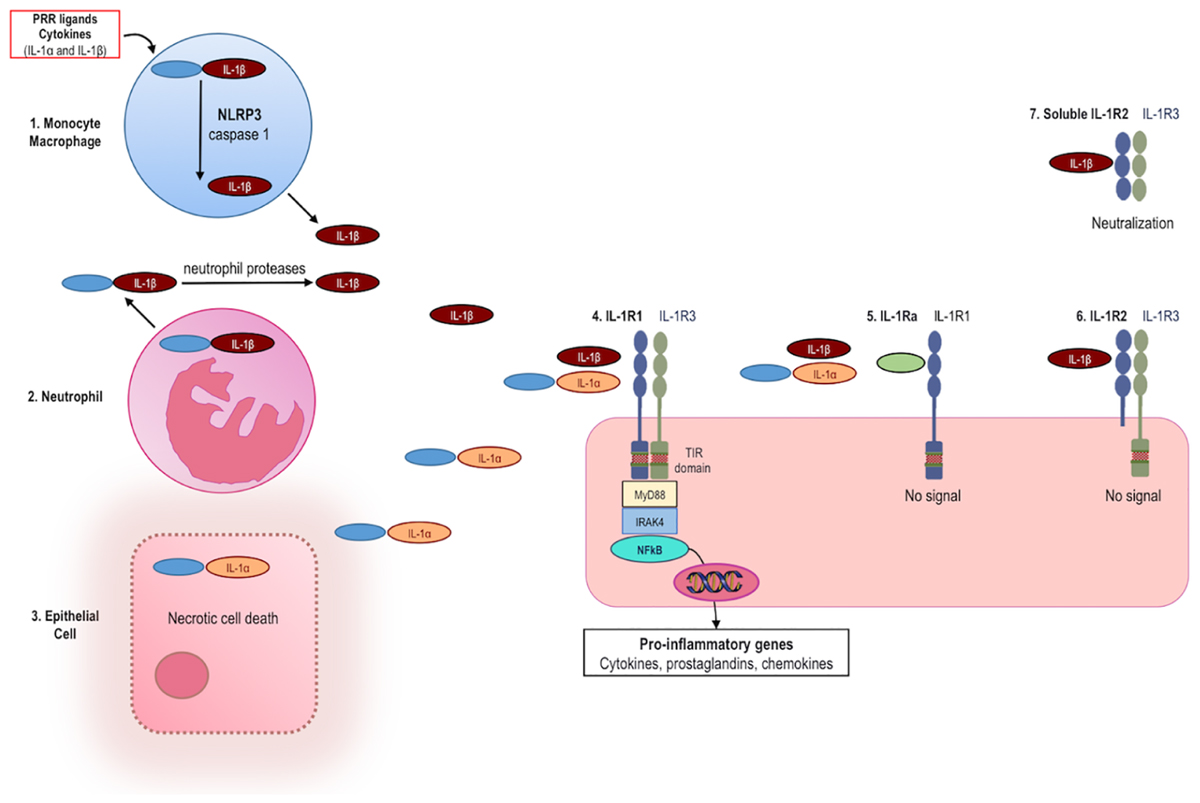 Frontiers Anakinra Therapy For Non Cancer Inflammatory Diseases Pharmacology
Frontiers Anakinra Therapy For Non Cancer Inflammatory Diseases Pharmacology
 Mechanisms Of Actions Of Anakinra Canakinumab Gevokizumab Download Scientific Diagram
Mechanisms Of Actions Of Anakinra Canakinumab Gevokizumab Download Scientific Diagram
 Inflammasome And Cytokine Blocking Strategies In Autoinflammatory Disorders Sciencedirect
Inflammasome And Cytokine Blocking Strategies In Autoinflammatory Disorders Sciencedirect
 The Long And Winding Road In Pharmaceutical Development Of Canakinumab From Rare Genetic Autoinflammatory Syndromes To Myocardial Infarction And Cancer Sciencedirect
The Long And Winding Road In Pharmaceutical Development Of Canakinumab From Rare Genetic Autoinflammatory Syndromes To Myocardial Infarction And Cancer Sciencedirect
 Current Understanding Of The Pathophysiology Of Systemic Juvenile Idiopathic Arthritis Sjia And Target Directed Therapeutic Approaches Sciencedirect
Current Understanding Of The Pathophysiology Of Systemic Juvenile Idiopathic Arthritis Sjia And Target Directed Therapeutic Approaches Sciencedirect
Strategies For Il 1 Blockade With Anakinra And Canakinumab A Download Scientific Diagram
Https Www Ema Europa Eu En Documents Variation Report Kineret H C 363 Ii 0056 Epar Assessment Report Variation En Pdf
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.