AVISE tests were developed and performance characteristics determined by Exagen Inc. AVISE Vasculitis AAV exemplifies the rigorous quality that is at the core of all AVISE testing products.
 Exagen Diagnostics Microsampling Method Gains Wider Acceptance
Exagen Diagnostics Microsampling Method Gains Wider Acceptance
Click here to learn how to access an AVISE test.
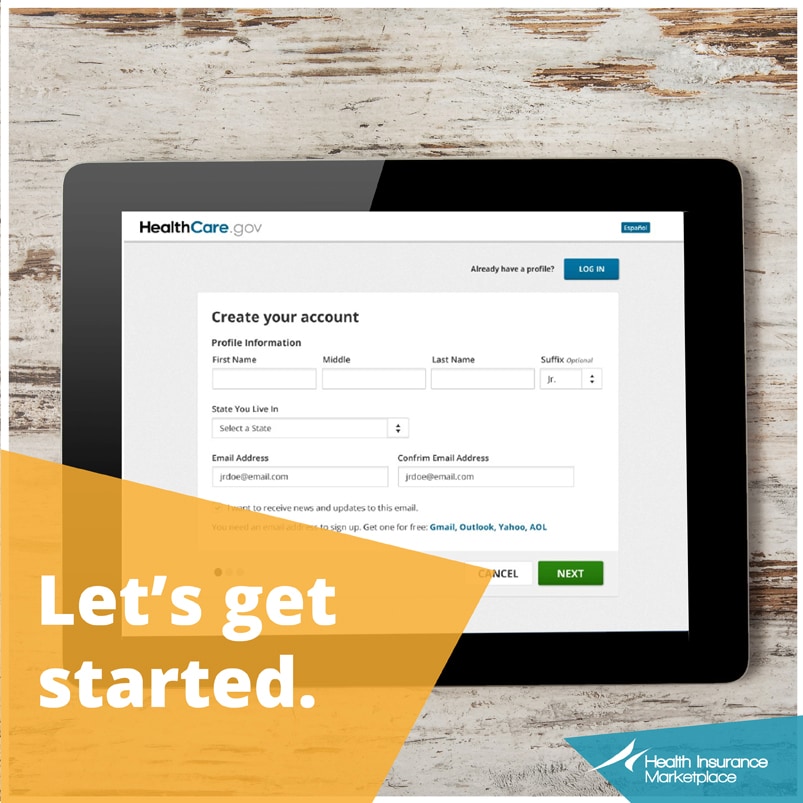
Exagen avise test. Is a patient-focused and discovery-driven commercial-stage life sciences company. To learn more about each individual test click the test name below. AVISE tests were developed and performance characteristics determined by Exagen Inc.
SAN DIEGO July 27 2020 GLOBE NEWSWIRE -- Exagen Inc. AVISE Tests Now Available to 6 Million Humana Military Beneficiaries. AVISE Lupus test is a 10-marker lupus diagnostic test containing Cell-Bound Complement Activation Products CB-CAPs and SLE associated markers designed to aid healthcare providers in timely differential diagnosis of SLE.
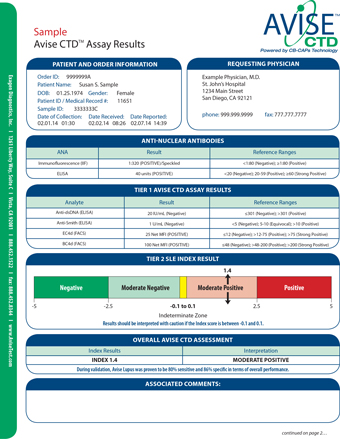
While some components of AVISE tests are FDA approved devices the integrative test methods have not been cleared or approved by the FDA. I just did a blood test that my rheumatologist said is new and can more specifically pin down what type of lupus you have. AVISE CTD contains patented biomarkers and algorithms to provide improved diagnostic information compared to traditional lab tests alone.
Specialized test to aid in the diagnosis and management of patients with antiphospholipid syndrome APS. AVISE CTD is an advanced autoimmune rheumatic disease test specifically designed to aid physicians in the differential diagnosis of systemic lupus erythematosus SLE. AVISE CTD is the only diagnostic test powered by patented Cell-Bound Complement Activation Products CB-CAPs stable biomarkers of complement activation to help physicians better assess patients.
SAN DIEGO Oct. Exagens Avise SLE Prognostic is a test which may be piggybacked with the Avise CTD to take the diagnosis a step further. 12 2020 GLOBE NEWSWIRE -- Exagen Inc.
Avise Exagen Test I was diagnosed with Lupus about three years ago based on symptoms and positive ANA. AVISE testing offers next-generation insight to aid the diagnosis prognosis and monitoring of autoimmune conditions. While some components of AVISE tests are FDA approved devices the integrative test methods have not been cleared or approved by the FDA.
Advertentie Oefen Hier Voor Het Officiële CBR Theorie-Examen Voor Auto Motor Of Bromfiets. The AVISE line includes diagnostic tests for lupus and antiphospholipid syndrome as well as for distinguishing between rheumatoid arthritis systemic lupus erythmatosus SLE fibromyalgia Sjögrens syndrome. This test also provides additional assessment for the potential risks for the involvement of the organs which could occur through a variety of different complications including cardiovascular events thrombosis neuropsychiatric Lupus and Lupus.
We are standing by to help with any AVISE testing needs or questions you may have. XGN an organization dedicated to transforming the care continuum for patients suffering from autoimmune diseases today announced that all AVISE test offerings CTD Lupus SLE Prognostic SLE Monitor. Advertentie Oefen Hier Voor Het Officiële CBR Theorie-Examen Voor Auto Motor Of Bromfiets.
Exagen is regulated under CLIA as qualified to perform high complexity testing. Please reach out to us at the contact information below and we will get back to you as soon as possible. We are committed to providing physicians with clinical tools to help address the significant unmet need for accurate and timely diagnosis prognosis and monitoring of autoimmune Connective Tissue Disease CTD.
Exagen Provider Relations Team. Is a commercial-stage life sciences company that develops and offers advanced autoimmune testing. XGN an organization dedicated to transforming the care continuum for patients suffering from autoimmune diseases today announced the publication of a study evaluating the economic benefits AVISE Lupus testing titled Evaluation of the Economic Benefit of Earlier Systemic.
The AVISE APS test is an advanced antiphospholipid syndrome APS diagnostic test composed of a combination of biomarkers including anti-phosphatidylserineprothrombin PSPT to help assess a patients risk for APS and thrombosis. The AVISE CTD test is an advanced blood test for lupus and connective tissue diseases. The advanced diagnostic test specifically designed to aid in the differential diagnosis of AAV.
Is committed to providing excellent service to our physicians patients and partners. Our relentless pursuit for laboratory excellence has enabled us to develop next-generation autoimmune testing to help empower physicians and improve patients lives. The Vasculitis AAV test adds to Exagens catalog of AVISE tests all aimed at the diagnosis prognosis and monitoring of autoimmune diseases.