The FDA however has already authorized three COVID-19 vaccines for emergency use. Currently no coronavirus vaccine is fully approved by the FDA but three were given emergency use authorization by the agency Published April 14 2021.
 Fda Approves Emergency Use Of A 2nd Covid 19 Vaccine
Fda Approves Emergency Use Of A 2nd Covid 19 Vaccine
Approval means the FDA has officially decided that a product is safe and effective for its.
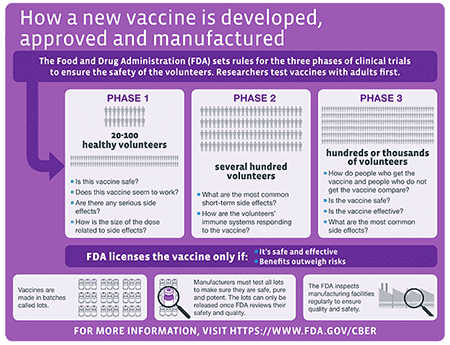
Does fda approve vaccines. Clinical trials are evaluating investigational COVID-19 vaccines in tens of thousands of study participants to generate the scientific data and other information needed by FDA to determine. Vaccines that appear promising in pre-clinical trials where the vaccine is tested on tissue samples and in animal models have to be approved for a clinical trial before they can be tested on humans. After its evaluation FDA decides whether to approve also known as to license the vaccine for use in the United States Applications designated as priority review mean the FDAs.
The FDA amended the emergency use authorization of the Johnson Johnson Janssen COVID-19 vaccine to include information about. April 23 2021. Its the first such.
Approval No COVID-19 vaccines have been approved. SAN DIEGO KGTV -- Pfizer could apply for full FDA approval of its COVID-19 vaccine as early as this month and Moderna could follow soon after. That timeline is first determined by the manufacturers of the products seeking FDA approval.
Food and Drug Administration FDA is the regulatory authority that has oversight of the safety effectiveness and quality of vaccines that are used in the United States. An FDA approval for a vaccine means the agency has decided that its benefits outweigh the known risks following a review of the manufacturers testing results. Currently the Pfizer vaccine has emergency approval from the FDA but full approval would allow Pfizer to market its vaccine directly to consumers.
FDA Sets Dates to Approve COVID-19 Vaccines While Pfizer Moderna Face Issues This picture taken on November 18 2020 shows a syringe and a. Vaccines as with all products regulated by FDA undergo a rigorous review of laboratory and clinical data to ensure the safety efficacy purity and potency of these products. The April 30 Facebook post features an image of Stephen Hahn who served as the FDA commissioner from December 2019 to January 2021 speaking.
If regulators sign off that status change would. If granted Pfizers full stamp of. The FDA amended the emergency use authorization of the Johnson Johnson Janssen COVID-19 vaccine to include information about a very rare and serious type of blood clot in people.
Vaccines must be FDA approved for clinical testing in humans. A more technical question that the FDA can help us answer is whether adenovirus vector vaccines if proven effective against initial infection can be used as boosters or for revaccination if. Food and Drug Administration ushered in a new phase of the fight against COVID-19 on Friday by giving its blessing to a vaccine made by Pfizer Inc.
Pfizer BioNTech Moderna and Johnson. Text in the photo reads FDA will not authorize or approve any COVID-19 vaccine. Pfizer is the first COVID vaccine maker to request full approval in the US and it will likely take several months for the FDA to review additional data and make an approval decision.
What will it take for the FDA to award them full approval and when can we expect that to occur - viewer Stephen asks. WBTV - There doesnt seem to be a lot of conversation about the fact that the FDA has only provided emergency authorization for current vaccines. 91 Zeilen April 23 2021.
A sponsor for such a clinical trial must submit an Investigational New Drug application to the FDA which describes the vaccine how. The FDA determines when a vaccine is FDA approved While the FDA determines if a vaccine qualifies for FDA approval and sets nonbinding guidelines for manufacturers to follow its not entirely up to the organization to decide when.